Graphene Film: Strong or Fragile?
Introduction
Graphene, the one-atom-thick film of pure carbon, is not merely a material; it's a scientific marvel that has garnered attention comparable to the invention of synthetic plastic. In this exploration, we unravel the intricacies of graphene's structure, its touted strength, the paradoxical fragility it exhibits, and the relentless pursuit of scalable production methods.
The Marvel of Graphene Structure
Graphene's hexagonal lattice, akin to honeycombs or chicken wire, conceals a world of remarkable properties. A single graphene flake, measuring a mere 1 nm in thickness, challenges our perceptions of thinness. Layers of graphene, when stacked, create graphite, a familiar material, while carbon fibers showcase a different structural approach, enhancing material intensity by preventing layer slippage.
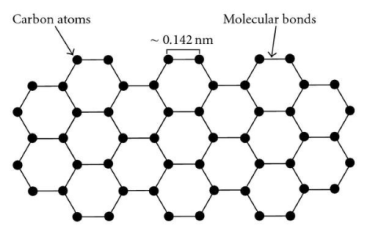
The Myth of Graphene Strength
Graphene's reputation precedes it as an exceptionally tough material. Claims of a graphene sheet supporting an elephant, though captivating, require a closer look. A 2008 research endeavor at Columbia University attributed graphene's strength to robust carbon-carbon covalent bonds and the absence of microscopic defects. While the theoretical intrinsic strength stands at 42 N/m, practical applications necessitate addressing microscopic defects like cracks and scratches.
The Fragility Dilemma
As we navigate the landscape of graphene, an intriguing paradox emerges: while possessing incredible intrinsic strength, graphene becomes more fragile as it grows larger. This fragility poses challenges for its application as a macroscopic material. The delicate balance between strength and vulnerability necessitates a nuanced approach in manufacturing and handling.
Advancements in Production Methods
The production methods of graphene play a pivotal role in realizing its potential. Chemical vapor deposition (CVD) stands out as a prominent technique, allowing the deposition of carbon atoms on a substrate, resulting in a higher quality but not without imperfections. Silicon-based epitaxy technology, announced in 2011, holds promise for large-scale, high-quality graphene production.
The Promise of Roll-to-Roll Manufacturing
In September 2013, Graphene Frontiers introduced a revolutionary roll-to-roll manufacturing technique, potentially revolutionizing large-scale production. While CVD offers quality, it tends to be expensive and impractical for commercialization. Graphene Frontiers' innovative approach aims to overcome these challenges, presenting an optimistic future for the mass production of high-quality graphene.
Conclusion
As we conclude our exploration into the realm of graphene, it's evident that this material is not just a scientific curiosity but a substance with transformative potential. From the hexagonal wonders of its atomic structure to the paradox of strength and fragility, graphene sparks both awe and challenge. In the relentless pursuit of large-scale, cost-effective production methods, Stanford Advanced Materials remains at the forefront, contributing to the journey that seeks to make graphene an economically viable and widely accessible material for various industries.