2 Different Ways to Produce Hyaluronic Acid and Its Applications
Introduction
Hyaluronic acid (HA), also called hyaluronan, is a linear polysaccharide that was generally discovered in 1918 by Karl Meyer and John Palmer [1]. Even now, HA still brings scientists lots of unconceived results. This article will give you a brief introduction to what hyaluronic acid is, how it is produced, and where it is used.
What is Hyaluronic Acid?
Hyaluronic acid (HA) is a linear glycosaminoglycan (GAG) consisting of repeating disaccharides of glucuronic acid-N-acetylglucosamine. See Figure 1. A general HA can repeat Figure 1’s structure more than 10000 times [2].
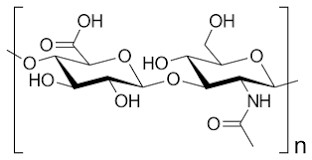
Figure 1: Structure of a hyaluronic acid monomer: Glucuronic Acid is on the left side and N-Acetylglucosamine is on the right side.
HA is often found in the extracellular matrix of epithelial, neural, and connective tissues of vertebrates. It is also found in some microorganisms, such as streptococcus, as one part of the extracellular capsule. Here, HA serves not only as a protector and an adhesive but also as a disguiser “deceiving” the host’s immune system during the infection process [2].
Because of its high moisture retention and good biocompatibility, hyaluronic acid is widely used in many areas of biomedical pharmaceuticals, cosmetics, and clinical studies. For example, hyaluronan can absorb large amounts of water and act as a lubricant and shock absorber in joints and connective tissues. Since hyaluronic acid is a repeating-structured compound, different repeating times give hyaluronic acid different properties, which means that HA can have more different applications and uses. Due to its increasing applications, the demand for HA continuously grows since its discovery up until now. People find two ways to obtain HA more efficiently and economically.
How is Hyaluronic Acid Produced?
Nowadays, there are two major sources to produce hyaluronic acid: animals and bacteria. Let’s first talk about hyaluronic acid produced from animal sources.
Hyaluronic Acid Produced From Animal Sources
Hyaluronic acid was first isolated from bovine vitreous humor by Meyer and Palmer in 1934. After that, many other animal tissues such as rooster comb, human umbilical cord, and bovine synovial fluid are used to extract hyaluronic acid. A total process involves animal tissue homogenization, extraction, purification, and final product preparation [1]. There are different extraction and purification methods for different feedstock and different grades of the hyaluronic acid final product. Here, let’s take high-purity HA for medical use as an example.
Washing: Before extraction, we need to “clean” our animal tissue with a mixture of ethanol and chloroform or with water, acetone, and ethanol. Animal tissue such as rooster comb is collected and treated with 95% ethanol desaturated with chloroform for one day. The washing step repeats several times till the washing solution is transparent and colorless. This is to make sure that all “bad” components, such as iron, copper, and phosphate ions, are washed away because HA can be oxidized or decomposed by them.
Extraction: Then, use water and chloroform (20:1) mixture to extract the already pretreated animal tissues, and stir at least 2 times to extract as many HA components as possible from the tissues. Let the extraction solution stand for at least 24h at 4 to 25℃. Filter the mixture solution, add chloride and chloroform (1:1), and stir for 3 to 5h at 4 to 25℃.
Purification: Since the HA is extracted from animal tissues, many impurities such as proteins, lipids, peptides or low molecular weight precursors exist in the extract. Some HA may even have covalent bonds with proteins or peptides. Simple organic solvent washing is not enough. Here, add the proteolytic enzymes to the extract solution at nature pH, and stir for 5 days at room temperature. Get the aqueous layer and use Teflon filters to sterilize the solution. Use 3 volumes ethanol to precipitate the polysaccharide. Last but not least, use ethanol and acetone to precipitate the polysaccharide again, sterilize it using acetone, and dry it under a vacuum condition [1].
The HA final product has a molecular mass larger than 750000 Da. The production efficiency is about 0.8g hyaluronic acid from 1kg of rooster combs.
Hyaluronic Acid Produced From Bacteria Sources
Hyaluronic acid produced from bacteria sources developed rapidly over the past 2 decades. There are a few numbers of gram-positive bacteria such as streptococcus or Pasteurella that can be used in making hyaluronic acid, and they usually need to meet the following requirements.
- These bacteria should be nontoxic or harmful to people and should not be haemolytic.
- These bacteria cannot show any hyaluronidase activity to HA [1]. HA is a long-chain compound with very simple duplication. HA is easily “broken” and affected by any mechanical, thermal, chemical, and enzymatic perturbations.
- High efficiency is also an important aspect to consider.
Group A and C Streptococci are the most popular and appropriate strains to produce HA due to their high yield. However, the general streptococcic group does produce toxins. The good news is that by discovering the gene code used in HA biosynthetic pathway, we can modify lots of bacteria (Bacillus, Agrobacterium, E. coli, Lactococcus) that can express the gene code and produce HA. In other words, we can first select non-haemolytic and hyaluronidase-negative bacteria, and then change some of their codes to produce HA [2].
The whole process to obtain hyaluronic acid from bacterial source is really complicated. Figure 5 shows how HA is produced by bacteria E. coli [2].
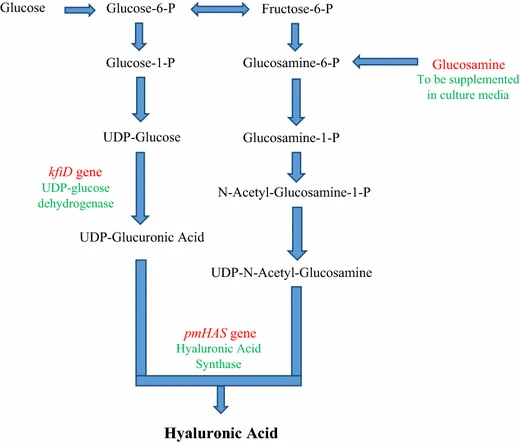
Figure 5: overview biosynthesis pathway of E. coli to produce hyaluronic acid [2]
Hyaluronic Acid Applications
Applications of Hyaluronic acid vary from its size: large molecule (about 1000~ 1500kDa), middle molecule (about 200~ 800kDa), and small molecule (about 5~ 200kDa).
Large molecule hyaluronic acid (HMW-HA) is often used in skincare products to help provide surface-level hydration to the skin. Because of its large molecular size, it is not able to penetrate deeply into the skin but instead forms a film on the surface of the skin to help prevent water loss and keep the skin hydrated. This type of HA is commonly found in moisturizers, sheet masks, and other hydrating skincare products.
Small molecule hyaluronic acid (LMW-HA), on the other hand, is able to penetrate more deeply into the skin. This makes it useful in anti-aging skincare products, where it can help to stimulate collagen production and improve the overall texture and appearance of the skin. It is also commonly used in serums and other lightweight formulations that are designed to be absorbed quickly by the skin.
Read more: High VS. Low Molecular Weight Hyaluronic Acid
Medium molecular weight hyaluronic acid (MMW-HA), as its name suggests, stands in the middle ground when it comes to molecule size. This type of HA is less commonly used in skincare products but is believed to have benefits for both surface-level hydration and deeper penetration into the skin. It may be useful in formulations that aim to provide both immediate and long-term hydration to the skin.
In addition to its use in skincare products, hyaluronic acid is also used in injectable treatments such as dermal fillers. These filters typically use a combination of large and small molecule hyaluronic acid to provide immediate plumping and hydration to the skin, as well as longer-lasting volumizing effects.
Overall, the different applications of hyaluronic acid depend on its molecular size and the specific skincare concerns it is being used to address. Large molecule HA is useful for surface-level hydration, small molecule HA is useful for anti-aging and collagen production, and medium molecule HA is less commonly used but may have benefits for both surface-level and deeper hydration.
Conclusion
In conclusion, hyaluronic acid can be extracted from animal sources and bacterial sources. And HA products of different sizes are used for different purposes in the skincare area.
Stanford Advanced Materials Company has rich experience in the manufacture and sales of high molecular weight, middle molecular weight, and low molecular weight hyaluronic acid. It’s important to choose a product that is well-formulated and suited to your specific skincare needs to ensure the best results. Please check our homepage for more information.
Reference
- Polyak, F., Khabarov, V. N., Boykov, P. Y., & Selyanin, M. A. (2015). Hyaluronic acid: Production, properties, application in biology and medicine. John Wiley & Sons, Incorporated.
- Sze, J. H., Brownlie, J. C., & Love, C. A. (2016). Biotechnological production of hyaluronic acid: a mini review. 3 Biotech, 6(1), 67. https://doi.org/10.1007/s13205-016-0379-9