An Overview of Calcium Carbonate Crystal Substrates
Introduction
Calcium carbonate crystal substrates emerge as a fascinating new frontier in material science. These substrates are gaining attention due to their unique properties and potential applications in various technological and environmental arenas.

This article discusses the characteristics, synthesis, applications, and future prospects of calcium carbonate crystal substrates, shedding light on their role in driving innovations in material science.
Characteristics of Calcium Carbonate Crystal Substrates
Calcium carbonate (CaCO₃) is a common substance found naturally in rocks, eggshells, pearls, and marine organisms like coral. In its purest form as a crystal substrate, calcium carbonate presents unique physical and chemical properties such as high biocompatibility, low thermal conductivity, and significant environmental stability. These properties make it an ideal candidate for various scientific and industrial applications.
 [1]
[1]
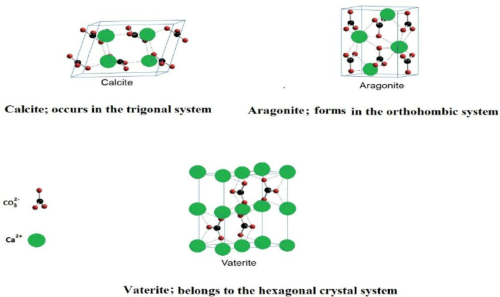
Calcium carbonate crystallizes in three polymorphic forms: calcite, aragonite, and vaterite. Each form offers different crystal structures and properties.
- Calcite is the most stable form. It exhibits excellent birefringence and is transparent to visible light, which is valuable in optics.
- Aragonite stands out for its high pressure and temperature stability. It is crucial in high-strength applications.
- Vaterite is less stable but is highly reactive. Thus, it is useful in rapid biomineralization processes.
Synthesis of Calcium Carbonate Crystal Substrates
The synthesis of calcium carbonate crystal substrates can be achieved through various methods, including chemical vapor deposition, hydrothermal synthesis, and biomimetic strategies.
- Chemical Vapor Deposition: This method allows for the formation of high-purity calcium carbonate by depositing vaporized compounds onto a substrate, creating thin films that are uniform and controllable.
- Hydrothermal Synthesis: Utilizing high pressures and temperatures, this method synthesizes crystals that are purer and have a more defined crystalline structure, ideal for high-performance applications.
 [2]
[2]
- Biomimetic Synthesis: Perhaps the most fascinating, this approach mimics biological processes to form crystals at ambient temperatures and pressures. It allows the integration of organic components, which can modify the properties of the resulting crystals for specific applications.
Among these, biomimetic synthesis is particularly intriguing because it allows the formation of crystals under mild conditions, mimicking natural processes. This method involves using organic molecules as templates to guide the crystal growth, resulting in highly controlled morphologies and sizes.
Environmental factors such as temperature, pH, and the presence of impurities or additives play crucial roles in determining the polymorphic form and quality of the crystals. For instance, the addition of magnesium ions can inhibit calcite formation, favoring the aragonite structure. Understanding these factors is essential for tailoring the synthesis process to produce desired crystal characteristics for specific applications.
Related reading: What is Chemical Vapor Deposition (CVD)?
Applications of Calcium Carbonate Crystal Substrates
Calcium carbonate crystal substrates have a wide array of applications due to their versatile properties.
- In Optics: The high birefringence of calcite, one form of calcium carbonate, makes it useful in the manufacturing of polarizers and waveplates, critical components in various optical instruments and devices.
- In Biomedicine: The biocompatibility and solubility of calcium carbonate make it suitable for drug delivery systems and as scaffolds in bone regeneration. It's particularly effective in controlled release formulations, providing a gradual release of therapeutics.
- In Environmental Science: Calcium carbonate's ability to absorb and mineralize CO2 has positioned it as a potential medium for carbon capture and storage (CCS) technologies aimed at reducing greenhouse gas emissions.
- Moreover, in the field of Catalysis, calcium carbonate substrates are used to develop new catalytic processes that are more efficient and less polluting.
Challenges and Future Prospects
Despite their potential, the use of calcium carbonate crystal substrates faces several challenges. The primary issue is the control of crystal purity and structure during synthesis, which is crucial for applications requiring high precision, such as in optics and electronics. Additionally, the scalability of synthesis methods needs to be addressed to meet industrial demands.
Looking forward, the research is geared towards improving the quality and control of synthetic calcium carbonate crystals and expanding their applications.
Advances in nanotechnology could lead to the development of nanostructured calcium carbonate substrates with enhanced properties such as increased surface area and reactivity, opening new possibilities in catalysis and environmental applications.
Another promising area is the integration of calcium carbonate substrates with other materials to create composite materials. These composites could combine the desirable properties of calcium carbonate with those of other materials, such as polymers or metals, to create supermaterials with novel properties for use in a wide range of applications.
Conclusion
Calcium carbonate crystal substrates represent a burgeoning field in material science. They offer remarkable opportunities due to their unique properties and broad applicability. As research continues to uncover the full potential of these materials, their impact on technology and environmental management is expected to grow significantly.
Table 1. Overview of Calcium Carbonate Crystal Substrates
|
Category |
Details |
|
Characteristics |
- High biocompatibility - Low thermal conductivity - Significant environmental stability |
|
Polymorphic Forms |
- Calcite: Stable, excellent birefringence, transparent to visible light - Aragonite: High pressure and temperature stability, suited for high-strength applications - Vaterite: Less stable, highly reactive, useful in rapid biomineralization |
|
Synthesis Methods |
- Chemical Vapor Deposition: High-purity, controllable thin films - Hydrothermal Synthesis: High pressure and temperature, defined structure - Biomimetic Synthesis: Mimics biological processes, allows integration of organic components |
|
Applications |
- Optics: Manufacture of polarizers and waveplates - Biomedicine: Drug delivery systems, bone regeneration scaffolds - Environmental Science: Carbon capture and storage (CCS), catalysis |
|
Challenges |
- Control of crystal purity and structure - Scalability of synthesis methods |
|
Future Prospects |
- Advances in nanotechnology for enhanced properties -Development of composite materials combining calcium carbonate with other materials for novel applications |
Stanford Advanced Materials (SAM) offers high-quality calcium carbonate crystals at competitive prices. As a leading supplier, SAM also provides over 3,000 advanced materials to major industries including aerospace, technology, medical, and energy. Customization is also available. Please check our homepage for more information.
Reference:
[1] Maleki Dizaj, Solmaz & Barzegar-Jalali, M. & Zarrintan, M. & Adibkia, Khosro & Lotfipour, Farzaneh. (2015). Calcium carbonate nanoparticles; Potential in bone and tooth disorders. Pharmaceutical Sciences. 20. 175-182. 10.5681/PS.2015.008.
[2] Tatarchuk, Tetiana & Peter, Amalthi & Al-Najar, Basma & Vijaya, Judith & Bououdina, Mohamed. (2018). Photocatalysis: Activity of Nanomaterials. 10.1002/9783527808854.ch8.