Satellite Phenomena in Metal Powder: A Deep Dive into Additive Manufacturing Challenges
1 Introduction
As an emerging manufacturing technology, additive manufacturing technology is widely used in aerospace, automotive, medical devices, and other fields that require high-precision processing. Compared with traditional manufacturing processes such as thermal spraying, the metal powder used in additive manufacturing needs to meet the requirements of small particle size, high sphericity, good fluidity, low oxygen content, and other characteristics.
Therefore, the preparation and development of special high-performance metal powders suitable for additive manufacturing is one of the keys to the development of additive manufacturing technology.

Fig. 1 Aerosolized powder-making equipment
2 Impact of Satellite Powder
2.1 Understanding Satellite Powder
Satellite powder is a defective powder formed when many small particles of powder adhere to the surface of a larger particle of powder. These particles are arranged around the base powder particles like the way satellites orbit planets. Such arrangement causes the larger particles to form one or more ring-like structures around the base powder, similar to the orbit of a planet around a star. For this reason, the phenomenon is referred to as satellite powder (See Figure 2. below).
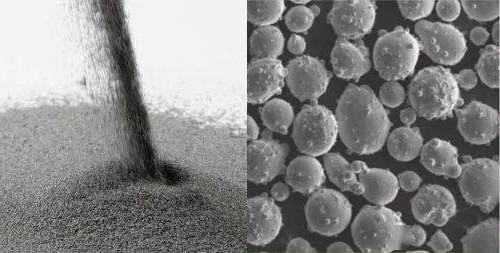
Fig. 2 Microscopic morphology of satellite powder
Satellite powder phenomenon usually occurs during powder metallurgy and powder metallurgical processing, which involves a variety of metal and alloy powders. Generally, satellite dust can occur with any metal powder used in powder metallurgy, but some metals or alloys may be more susceptible to the phenomenon, depending on their properties and the conditions during preparation. Here are some types of metals that may be more susceptible to satellite powders.
1. Iron and iron-based alloys: Micro Iron Powder, Ferro Tungsten Powder, Ferro Niobium Powder
2. Cobalt and its alloys: Micro Cobalt Powder, Tungsten Carbide/Cobalt/Chromium Powder, Cobalt-based Alloy Powder (Co-Cr-Mo)
Besides, Nickel, Titanium, Aluminium, and their alloy powders are also used more in the metallurgical industry and may be affected by satellite powders.
2.2 Hazards to Metal Powder Production
The presence of satellite powders reduces the bulk density, sphericity, and flowability of metal powders, which is detrimental to the powder lay-up process. It also has a non-negligible impact on metal additive manufacturing processes (especially some processes based on powder lay-up techniques).
In addition, as satellite powders are formed, larger powder particles tend to tend to cluster together due to adsorption and surface tension between particles. This adsorption and surface tension can cause the satellite powder to adhere closely to the base powder particles, increasing the difficulty of separation. This defective powder is therefore difficult to remove effectively utilizing subsequent treatment and its formation needs to be controlled at source.

Fig. 3 Micro Iron Powder
3 Causes of Satellite Powder
3.1 Causes of Powder Agglomeration
The satellite powder phenomenon generated during metal powder processing is closely related to the inherent nature of the powder particles themselves. The reasons for its formation mainly include mutual attraction between powder particles, particle inhomogeneity, and differences in distribution and density.
1. Mutual attraction between powder particles: In the powder bed, there may be a certain degree of attraction between the metal powder particles, resulting in their aggregation to form satellite powder.
2. Uneven shape and size of powder particles: If the shape and size of the metal powder are not uniform, some of the larger particles may attract the smaller particles around them, forming satellite powders.
3. Differences in powder distribution and density: There may be uneven density in the powder layer, resulting in more powder build-up in some places than others, thus forming satellite powders.
These three causes are essentially summarized as mutual attraction between powder particles, which causes the powder particles to attract and aggregate with each other to form satellite powder.
3.2 Factors related to equipment
The occurrence of satellite powder is closely linked to the equipment and facilities used in the production of spherical metal powders. Illustrated in the provided image is a model of an atomization chamber, complete with boundary condition setups that underscore the crucial environment and parameters pivotal to spherical metal powder production.
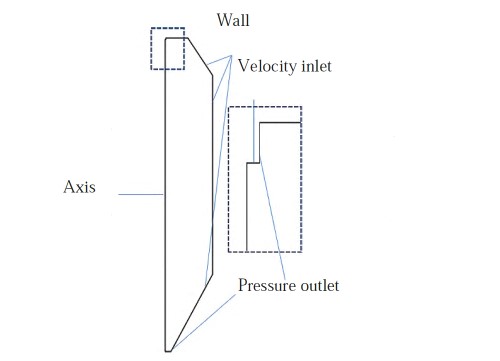
Fig. 4 Atomization chamber model and the boundary condition setup
It has been studied that the closed structure of the atomization chamber generates macro-scale vortices near its sidewalls, known as gas recirculation (GR), which entrain some fully solidified small-sized particles. The collision between the small-sized particles rising back in the recirculation zone and the incompletely solidified large-sized droplets in the upstream atomized gas stream is one of the main causes of satellite powder formation.
Therefore, taking gas rectification measures to limit the dust gyration caused by reflux becomes an effective means to control the formation of satellite powder on a macro scale. At present, the gas rectification measures for the control of satellite dust include the imposition of auxiliary airflow [9,10], and the improvement of the structure of the atomization chamber.
4 Strategies for Mitigating Satellite Powder Formation
--Optimizing Gas Incidence Angle for Dispersion
Satellite powder defects are mainly formed in the secondary atomization stage, where small-sized droplets, due to their large specific surface area and high cooling rate, first solidify and collide with large-sized droplets that have not yet fully solidified, and eventually attach to the surface of large-sized particles, forming satellite powder. Therefore, one basic idea is to make the droplets fully dispersed by changing the angle of gas incidence, which can effectively reduce the generation of satellite powder.
--Adjusting Solidification Timing and Particle Concentration
Another idea is to change the solidification time of the powder and the concentration of the particles in various states by controlling the atomization rate and the pressure of the atomization chamber while keeping other conditions constant, to achieve the problem of reducing the collision between particles and the adhesion of fine powders. With the reduction of atomization rate, the solidification time of powder is shortened, which can effectively reduce the phenomenon of powder adhesion; with the reduction of pressure in the atomization chamber, the concentration of fine powder in the atomization chamber gradually decreases, so the chance of collision between the powders also decreases, which improves the sphericity of the powder.
--Inhibiting Reflux Gas to Prevent Powder Recirculation
Through the observation of the aerosolization process, it was found that a part of the small particles of powder would flow upwards with the reflux gas, re-enter the atomization area, and collide with the liquid droplets that had not yet solidified to form satellite powder. By inhibiting the reflux gas, the phenomenon of fine powder reflux can be avoided to reduce the satellite powder.
--Employing Auxiliary Airflow to Suppress Backflow
Additionally, the reduction of satellite dust generation can be achieved by adding an auxiliary airflow to suppress backflow. When an auxiliary airflow with an auxiliary mist ratio of >0.8 is applied at 1/2R from the center of the chamber, the auxiliary airflow can effectively inhibit the cyclone of dust. Besides, the stepped atomization chamber structure can effectively suppress dust cycling when the step size is D=300 mm, ΔH=575-600 mm, and the step angle is moderate (See Table below) [14].
Table 1 Morphological characteristics of the powder samples
|
Sample |
Sphericity |
Redundancy Index |
|
TC4-1 |
0.9278±0.0311 |
0.489±0.062 |
|
TC4-2 |
0.9427±0.0165 |
0.270±0.027 |
5 Conclusion
Satellite powder formation in the additive manufacturing (AM) process is a critical issue affecting the quality of metal powders. The presence of satellite powder will reduce the loose loading density of metal powder, sphericity, and mobility, but is not conducive to the process of powder laying.
In the process of aerosolization, the collision between the small-sized particles cycling up in the reflux zone and the incompletely solidified large-sized droplets in the upstream aerosolized gas stream is one of the main reasons for the formation of satellite powder. Equipment-related factors, such as the design of the atomization chamber and the conditions within, also play a significant role.
In this regard, inhibiting the return gas, such as adding an auxiliary gas stream is an effective solution. Strategies to mitigate satellite powder formation also include optimizing gas incidence angles, adjusting solidification times and particle concentrations, and employing auxiliary airflow. These measures aim to improve powder quality by reducing undesired agglomeration and enhancing the manufacturing process's efficiency and reliability in industries like aerospace, automotive, and medical devices.
Stanford Advanced Materials (SAM) offers a range of spherical powder products for purchase. Our focus is on the research and development, manufacturing, and sales of high-quality spherical powders. SAM also provides personalized customization services to meet specific customer needs. Send us an inquiry if you are interested.
Reference:
[1] Fuzhong Chu, Kai Zhang, Haopeng Shen, Meijuan Liu, Wenjing Huang, Xi Zhang, Enquan Liang, Zongyan Zhou, Liming Lei, Juan Hou, Aijun Huang, Influence of satellite and agglomeration of powder on the processability of AlSi10Mg powder in Laser Powder Bed Fusion, Journal of Materials Research and Technology, Volume 11, 2021, Pages 2059-2073, https://www.sciencedirect.com/science/article/pii/S223878542100140X
Related Link:
https://www.sciencedirect.com/science/article/pii/S223878542100140X